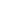Understanding Metal Melting Points: A Comprehensive Guide for Manufacturing Professionals
 May 13,2024
May 13,2024

This article will highlight the importance of metal melting points in manufacturing processes. The lowest melting point in metals is mercury which is -38 degrees Celsius. The highest melting point is of tungsten which is 3422 degrees Celsius. This shows that metal melting points significantly vary, that’s why some metal manufacturing is cost-effective while others are very expensive because it needs more heat energy to manufacture them. Choosing appropriate post heat treatment and manufacturing process requires the information of melting point of metals. This article will help you to understand the impact of metal melting points in production processes, phase transition, thermal conductivity, thermal expansion, and specific heat of the metals.
Fundamental Concepts of Metal Melting Points
Phase change of metal from solid state to liquid state occurs at melting point. All metal's melting points are their fundamental property vary from metal to metal. Alloying metals shift their melting temperature depending upon the alloying element.
YouTube video explains the melting point of different metals.
https://www.youtube.com/watch?v=q198D12MhNw&pp=ygUXbWVsdGluZyBwb2ludCBvZiBtZXRhbHM%3D
Understanding Melting and Boiling Points
- States of Matter and Phase Transitions
All matter has 3 states in which they exist naturally solid state, liquid state, and gas state. The change of matter from one phase to another is called phase transition.For example, all metals are changed from a solid state to a liquid state for shaping them into a particular shape.
Image showing three states of matter.
- Boiling Point vs. Melting Point
The conversion of liquid phase to gaseous state happens at boiling point. The conversion of solid phase to liquid phase occurs at melting point. Boiling points are in general greater than melting points.
Thermal Properties of Metals
- Thermal Expansion
The property of metal to expand and contract when exposed to temperature changes is called thermal expansion.
Image showing different thermal expansion in metals.
YouTube video showing the thermal expansion of the metal.
https://www.youtube.com/watch?v=82FPQ6z8vcE&pp=ygUbdGhlcm1hbCBleHBhbnNpb24gb2YgbWV0YWxz
- Thermal Conductivity
The ability of metal to conduct heat from high to low temperature difference is called thermal conductivity.
|
Metal |
Coefficient of Thermal Expansion (CTE) (μm/m°C) |
Thermal Conductivity (W/m°C) |
|
Aluminum |
23.1 |
237 |
|
Copper |
16.6 |
401 |
|
Steel (Carbon) |
10.8 |
50 - 60 |
|
Stainless Steel |
16.0 |
14 - 45 |
|
Titanium |
8.6 |
21.9 |
|
Nickel |
13.3 |
90 - 100 |
|
Lead |
29.0 |
35.3 |
|
Zinc |
30.2 |
116 |
|
Brass |
19.0 - 20.0 |
109 - 125 |
|
Iron |
11.8 |
80 |
|
Silver |
19.7 |
429 |
|
Gold |
14.2 |
318 |
|
Platinum |
8.8 |
71.6 |
Table showing coefficient of thermal expansion and conductivity of different metals.
YouTube video explaining the thermal conductivity of metals.
https://www.youtube.com/watch?v=qliCbHSEICQ
3 Factors Influencing Metal Melting Points
- Chemical Composition and Alloying
Chemical compositions and metal alloying can significantly alter the melting point of metals. It may decrease or increase the melting temperatures of metals. In metal alloys, the alloying element changes the bonding and arrangement of atoms inside the metal lattice therefore increasing or reducing the required energy for melting the metal.
- The Role of Impurities
The impurities also significantly reduce or increase the melting temperature for example presence of Sulphur and phosphorus in steel significantly decreases its melting point.
- Grain Structure and Heat Treatment
The grain structure of metals helps us to determine the heat treatments and cooling rate of the metals after manufacturing. Fine grains have a high melting point due to the uniform arrangement of atoms in the crystal lattice and are brittle and hard. The martensitic microstructure has a high melting point with fine grain structure. While coarse grain structure has a low melting point and is ductile.
Detailed Analysis of Specific Metals
The detailed analysis of specific metal is given below on behave of their melting points.
Copper and Its Applications
- Melting Points of Copper
The melting point of copper is 1,083°C which is the highest temperature enough for copper to maintain its structural integrity against resistance heat produced during conduction.
- Electrical Wiring and Copper's Thermal Properties
Copper is one of the best-known electric conductors, that’s why it is used in electrical wiring. At 20 degrees Celsius, the conductivity of copper is 58.5×106 (S/m). Copper also has good thermal conductivity making it ideal to use in electrical conduction applications because it would transfer heat which protects components from overheating. The ductility of copper makes it easy to wire conversion.

Image showing copper electrical wires.
- Applications
Some applications of copper are given below.
- Electrical Wiring
- Conductors
- Electronics
- Heat Exchangers
- Cooling system
- Architecture
- Cookware
- Decorative Items
- Medical Equipment
- Musical Instruments
Steel and Its Variants
One of the types of metal alloys is steel and its variants. Steel is the metal alloy of iron and carbon. The different steel grades melting points, applications and characteristics are discussed below.
|
Steel Grade |
Melting Point (°C) Range |
Characteristics |
Common Applications |
|
Carbon Steel |
1,370 - 1,480 |
Iron Carbon |
Structural components Machinery Pipelines |
|
Stainless Steel 304 |
1,370 - 1,420 |
Chromium Nickel |
Food processing Medical devices Utensils |
|
Stainless Steel 316 |
1,370 - 1,420 |
Chromium Nickel Molybdenum |
Chemical industry Marine applications |
|
Tool Steel |
1,370 - 1,480 |
Tungsten Molybdenum Vanadium |
Cutting tools Molds Dies |
|
Alloy Steel |
1,370 - 1,480 |
Additional alloying elements (like silicon and manganese) |
Automotive parts Aerospace components |

Image showing different grades of steel.
Aluminum and Its Alloys
- Melting Point of Aluminum and Its Use in Industry
Aluminum is soft and has ductility in nature, that's why has the lowest melting point among other metals. Aluminum is lightweight due to its low density. It is used in aerospace applications. The melting point of aluminum is 660 degrees Celsius. Aluminum is used in different applications listed below.
- aircraft structures like fuselage.
- automotive industry like body panels, and wheels.
- packaging materials.
- construction materials.
- Aluminum Alloys: Varieties and Melting Ranges
| Aluminum Alloy Grade | Melting Range (°C) | Melting Range (°F) | Characteristics and Applications |
|---|---|---|---|
| 1050 | 643 - 657 | 1189 - 1215 | High purity, excellent corrosion resistance, high thermal conductivity, commonly used in chemical and food processing |
| 1060 | 646 - 657 | 1195 - 1215 | Similar to 1050, slightly better strength, good formability, used for reflectors, decorations, and heat sinks |
| 1070 | 646 - 658 | 1195 - 1216 | Very high purity, excellent workability and weldability, used in electrical and chemical applications |
| 2014 | 507 - 638 | 945 - 1180 | Strong, used in heavy-duty structures requiring good strength to weight ratio, aircraft, military vehicles and bridges |
| 2017 | 502 - 638 | 936 - 1180 | A variant of 2014 with slightly different characteristics, mainly in military and aerospace applications |
| 2024 | 502 - 638 | 935 - 1180 | High strength, used in aircraft structures and hardware due to good fatigue resistance |
| 3003 | 644 - 654 | 1190 - 1220 | Good workability, weldability, and corrosion resistance, used in cookware and general sheet metal work |
| 3005 | 632 - 655 | 1170 - 1210 | Improved strength over 3003, better corrosion resistance, used in high moisture environments |
| 5052 | 607 - 650 | 1125 - 1202 | High strength, excellent corrosion resistance, particularly in marine environments, used in shipbuilding |
| 5083 | 570 - 640 | 1058 - 1184 | Very strong, corrosion-resistant, excellent for welding, used in marine, automotive, and aircraft applications |
| 6061 | 582 - 652 | 1080 - 1205 | Versatile, good mechanical properties, corrosion resistance, used in construction, aerospace, and automotive industries |
| 6063 | 600 - 650 | 1112 - 1202 | Great for extrusion, good strength and surface finish, used in architectural applications and window frames |
| 6082 | 555 - 650 | 1030 - 1202 | Similar to 6061 with better corrosion resistance and strength, used in structural applications and transport |
| 7075 | 477 - 635 | 890 - 1175 | Very high strength, used in aerospace and military applications due to excellent strength to weight ratio |
Table showing aluminum metal alloys and their respective melting points.
Brass and Other Copper Alloys
- Brass Melting Point and Usage in Manufacturing
Copper metal alloys include brass and bronze. Brass has a melting point of 1700° F (927°C). Metal alloy brass is a mixture of copper and zinc. It is used in applications like decorative items, kitchen utensils, fittings, and musical instruments.
- Copper Clad and Other Copper-Based Metals
Cladding-based copper has excellent thermal conductivity and electrical conductivity which is why used in applications like electric wiring, heat sinks, circuit applications, telecommunications, and automotive industries. Cladding are composite material in which the copper layer is bound to different substance materials. Bronze is a metal alloy of copper and tin with a melting point of 950 degrees Celsius. Bronze is used in applications like electrical connectors, bushings, springs, bearings, and musical instruments.

Image showing copper cladding used for construction purposes.
Metal Melting Points Chart
Different metals have different bounding energy with atoms in their crystal lattice that is why require different temperatures to break them up. From the chart below it can be seen that melting point of mercury is lowest and the melting point of tungsten is highest. From the data below it is included that less amount of energy is required to convert a solid to a liquid phase in mercury while a lot of energy is required for the phase transition of tungsten.
|
Metal |
Fahrenheit (°F) |
Celsius (°C) |
Kelvin (K) |
|
Mercury |
-37.9 |
-38.8 |
234 |
|
Gallium |
85.6 |
29.8 |
302.9 |
|
Cesium |
83.3 |
28.5 |
301.7 |
|
Rubidium |
102.9 |
39.4 |
312.5 |
|
Potassium |
146.1 |
63.4 |
336.5 |
|
Sodium |
208.0 |
97.8 |
370.9 |
|
Tin |
449 |
232 |
505 |
|
Zinc |
787 |
419 |
692 |
|
Cadmium |
610 |
321.1 |
594.3 |
|
Lead |
621 |
327 |
600 |
|
Aluminum |
1,221 |
660 |
933 |
|
Silver |
1,763 |
961 |
1,234 |
|
Gold |
1,945 |
1,064 |
1,337 |
|
Copper |
1,984 |
1,083 |
1,356 |
|
Nickel |
2,651 |
1,455 |
1,728 |
|
Iron (Steel) |
2,797 |
1,535 |
1,808 |
Table showing melting points of all metals stating from lowest to highest melting point.
Practical Applications in Industry
Metals with high temperatures of melting can be used in applications where material must face high temperatures throughout its service life. Engine components and furnace components can be manufactured with metals that have the highest melting point among other metals. Other metals with low melting points can be used in structural components, kitchen utensils, and construction applications where they don't have to face high temperatures in their service life.
Manufacturing Processes Involving Melting
- Smelting Techniques and Their Efficiency
In smelting metal ore is melted at high temperature with a reducing agent for metal extraction purposes. The phase transition of ore from solid to liquid form would happen. Different types of smelting include:
Blast furnace smelting.
Iron is extracted from its ore by using coke as a reducing agent. This process is highly efficient for large-scale production of iron.
Electric Arc Furnace.
In this scrap of steel is converted to liquid state at high temperature. This liquid state should be suitable for casting. This method is more flexible and efficient compared to the typical blast furnace method.

Image showing smelting of metal ore.
YouTube video showing the smelting of iron.
https://www.youtube.com/watch?v=9l7JqonyoKA&pp=ygUUc21lbHRpbmcgcHJvY2VzIGxpdmU%3D
- Jeweler Casting Supplies and Techniques
- Jeweler casting supplies include ceramic or graphite crucible, mold, investment material (gypsum-based investment powders that can attain high temperatures), and casting tools.
- Jeweler casting techniques include lost-wax Casting, Centrifugal Casting, Vacuum Casting, and Sand Casting.

Image showing cast jeweler.
YouTube video showing the jewelry casting process.
https://www.youtube.com/watch?v=oJ8ES25OpT8&pp=ygUQandld2xlcnkgY2FzdGluZw%3D%3D
Melting Points and Product Quality
- Quality Metal Products and Melting Standards
The quality metal product can only be obtained if you stick to the specific melting standards for the melting process. A quality product can only be obtained if you follow metal standards like temperature control, alloy composition, impurity control, and heat treatment. Product quality considerations involve homogeneity of alloy metals, avoiding oxidation, inclusion control, and retaining metallurgical integrity.
- Component Failure and Melting Point Considerations
Component failure would occur if the proper consideration of the melting point of the metal is not done for the application. The low melting point metals cannot be used in the component that has to face high temperatures in their service life because the material would not have enough creep to work in that environment and would ultimately fail.
Melting Points About Various Industries
Melting Points in the Automotive Industry
- How Does a Car Work: Metals in Engines
Engines in cars must face high temperatures and combustion stresses so the material chosen should retain good mechanical properties at high temperatures. Steel with a high melting point of 1,370°C and cast iron with a high melting point of 1,370 °C are used for this purpose.
- Melting Points of Metals in Electric Arc Engines
Electrical car engines require material that has excellent electrical conductivity and thermal conductivity. Copper with a melting point of 1,083°C and aluminum with a melting point of 660°C are best candidates for this purpose.
Melting Points in Everyday Products
- Metal Cans: Low Melting Point Metals
The metal used for can making is aluminum with a low melting point of 660°C. The low melting point of aluminum makes its fabrication cost-effective, proves its ductility nature, and can be recycled easily making it the best candidate for this use.

Image showing aluminum cans.
- Metal Glue and Liquid Phase Metals
Low-melting metals and solder alloys are used to join two metals or materials. Tin-lead solder is one example of many solders which have a low melting point of 180°C. these solder metal alloys have the lowest melting point making them perfect for the metal glue application.

Image showing the soldering process.
YouTube video showing the soldering process.
https://www.youtube.com/watch?v=6rmErwU5E-k&pp=ygUJc29sZGVyaW5n
Advanced Topics in Metal Melting Points
Metals with Extreme Melting Points
- Which Metal Has the Highest Melting Point?
Tungsten has the highest melting point of 3,422°C (6,192°F). Due to the high-temperature melting of tungsten, it is used in applications like light bulb filaments and industrial tools.
- Metals with Low Melting Points: Applications and Uses
Mercury has the lowest melting temperature of -37.89°C. Due to its low melting temperature, it is used in scientific instruments, dental amalgams, electrical switches, and relays. Tin also has a low melting point of 232°C, which is why used in applications like solder formation.
The Science of Melting Metals
- How to Melt Metal: Techniques and Precautions
Techniques for melting metals include induction heating, oxy-fuel combustion, blast furnace melting, and electric arc furnace (EAF). The following precautions must be taken when melting a metal.
- Wear safety gear like gloves, heat resistance clothes, safety shoes, and eye protection.
- Do melting in ventilated areas to prevent fumes and gas accumulation.
- Flexure is used to remove oxidation and melting impurities.
- Temperature control should be done to ensure proper melting[26].
- Liquid State and Pressure Canning of Metals
The liquid state of metal allows the metal to flow this is done for casting purposes normally. The metal is melted beyond its melting point to have a liquid state that can flow. Pressure canning of metal is done to alter the physical properties of metal by applying temperature or pressure. Heat treatments like quenching, annealing, normalizing, and tempering are considered in it. This process may enhance the hardness or ductility of the material depending upon the process.
Conclusion
We can conclude that the melting point is the fundamental property of all metals. All applications require a material with a specific melting point. The appropriate selection of metal for high-temperature application is required otherwise failure of metals will happen. The melting point of the metal depends upon alloying elements, some may increase the melting points other may decrease the melting point.
FAQ
What melts metal?
Heating metals to high temperatures allows the breaking of internal bonds and causes the phase transition of metals from a solid to a liquid phase.
Melting point of wood
Wood doesn’t have a melting point because it’s an organic component and when subjected to high temperature it undergoes thermal degradation.
At what temperature does steel boil?
Steel does not go from liquid to solid phase instead it changes its phase from solid to vapor at 2,800°C (5,072°F)
Which metal has the lowest melting point?
Mercury has the lowest melting point of -38°F (-39°C).
 Tel/WeChat:
Tel/WeChat:  Email:
Email: 
 Home
Home
 Stainless Steel Melting Point: An Essential Guide for Mechanical Engineers
Stainless Steel Melting Point: An Essential Guide for Mechanical Engineers