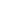91 Types of Metals and Their Engineering Applications
 Apr 03,2024
Apr 03,2024

Metals are the backbone of engineering they are used for manufacturing the structure of skyscrapers to cutting edge machinery. In mechanical engineering, metals are profound due to their significance in durability, innovation, high melting point, high strength-to-weight ratio, and efficiency across various industries. In mechanical engineering materials metals are used in the construction of structural components, machinery, and tools due to their remarkable properties making them suitable for engineering material applications. In this article, you will get an in-depth analysis of the properties of metals, reactions of metals, metal fabrication, bonding they participate in, and use of metals in different engineering applications.
Fundamental Concepts of Metals
Metals are natural in organic components. Metal fabrication is done by extracting from the raw material (ores) found on the Erath surface. Metals are best known for their properties like high melting points, durability, luster, and high strength to weight ratio. Metals are good conductor of heat and electricity.
Defining Metals: From Atomic Structure to Practical Uses
All kinds of metals are composed of atoms. Atoms are the smallest structures that are held together strongly by metallic bonding also known as delocalization bond. The simplest structure atom is the building block for all kinds of metals. Practically understanding the atomic structure of different types of metals will help you to understand material engineering and the reactivity of metals.
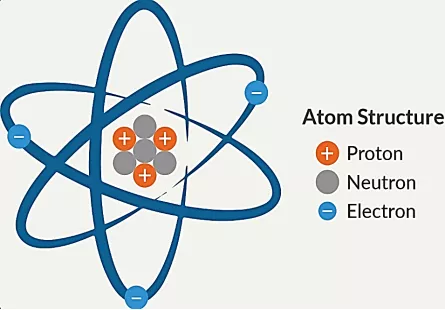
Figure showing the structure of the atom.
Atomic Structure and Electron Cloud Model
Atoms consist of the nucleus in the middle containing protons (positively charged) and neutrons. This nucleus is surrounded by the electron cloud. While electron cloud system describes the probability zone for electron location around the nucleus due to the uncertainty principle. To comprehend the behavior of different types of metals in terms of bonding, properties, and reactivity in different environments it is crucial to understand the electron cloud model.
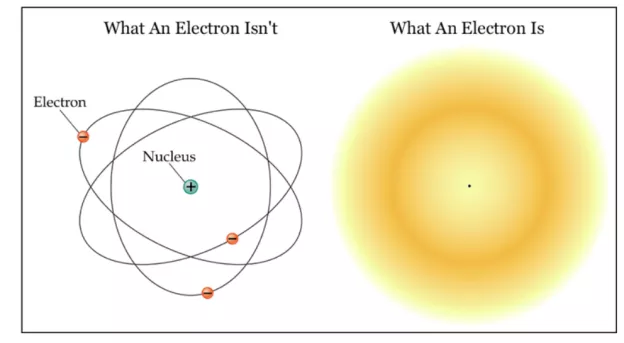
Figure illustrating the difference between Bohr Atomic Model and Electron Cloud Model.
YouTube video explaining the electron cloud model.
https://www.youtube.com/watch?v=FMlZq9NG_lo&pp=ygUjYmhvciBhdG9taWMgbW9kZWwgdnMgZWxlY3Ryb24gY2xvdWQ%3D
Metals on the Periodic Table
What is a metal on the periodic table
A significant portion of the periodic table is occupied by precious metals. Metals are typically found at the left side and middle of the periodic table. Hydrogen is not included as metal. Metals tend to lose electrons making them self-positively charged ions that form metallic bonding. There are almost 91 total metals in the periodic table.
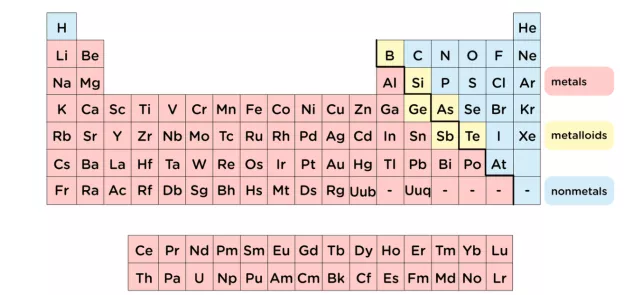
Periodic table showing the position of metals, metalloids, and nonmetals on the periodic table.
YouTube video explaining the difference between metals, metalloids, and nonmetals.
https://www.youtube.com/watch?v=dpyfCuXVSkg&pp=ygUfbWV0YWxzIG5vbm1ldGFscyBhbmQgbWV0YWxsb2lkcw%3D%3D
Basic Properties and Types of Metals
1. Physical Properties: Melting Point and Electrical Conductivity
Metals have high melting points and are good electrical conductors.
| Metal | Melting Point (°C) | Electrical Conductivity (S/m) |
| Copper (Cu) | 1083 | 5.96 x 10^7 |
| Aluminum (Al) | 660 | 3.77 x 10^7 |
| Iron (Fe) | 1538 | 1.0 x 10^7 |
| Silver (Ag) | 961 | 6.30 x 10^7 |
| Gold (Au) | 1064 | 4.10 x 10^7 |
| Nickel (Ni) | 1455 | 1.43 x 10^7 |
| Titanium (Ti) | 1668 | 1.23 x 10^6 |
Table showing melting point and electrical conductivity of metals.
Chemical Properties: Reaction with Oxygen and Corrosion Resistance
1. Reaction with Oxygen:
Raw materials of metals are reduced to form pure metals. Normally metallurgy of metals is that they react very quickly with oxygen because all type of metals tries to regain their raw material form of oxides. This reaction of all kinds of metals with oxygen is known as oxidation. The process of oxygen reaction is known as oxidation.
For example
- Iron oxide production happens when iron reacts with oxygen. Below is the reaction of oxygen with iron 4Fe+3O2→2Fe2O3

Figure showing iron rusting phenomena.
- Aluminum oxide is formed when aluminum (Al) reacts with oxygen 4Al+3O2→2Al2O3
- Copper oxide is formed when Copper (Cu) reacts with oxygen 4Cu+O2→2Cu2O
YouTube video explaining the process of iron rusting and its prevention.
https://www.youtube.com/watch?v=jQoE_9x37mQ&pp=ygUMaXJvbiBydXN0aW5n
2. Corrosion Resistance:
Corrosion resistance is the ability of precious metals to withstand chemical reaction deterioration in different environments. There are some metals that show excellent corrosion resistance due to the formation of oxide passivation layer. Normally metals with high electronegative value in the Pauling scale have a more stable oxide layer which improves their corrosion resistance compared with low electronegative metals. For example, the precious metal gold has more electronegative value on the Pauling scale that's why has a better electronegative value compared to Fe and Zn.
| Metal | Electronegativity |
| Aluminum | 1.61 |
| Copper | 1.90 |
| Iron | 1.83 |
| Zinc | 1.65 |
| Gold | 2.54 |
Table showing electronegativity of some metals from Pauling scale.
According to the principle of metallurgy when two metals form a galvanic cell the metal with high potential will act as an anode in a cell and hence corrode while the other metal would be protective.
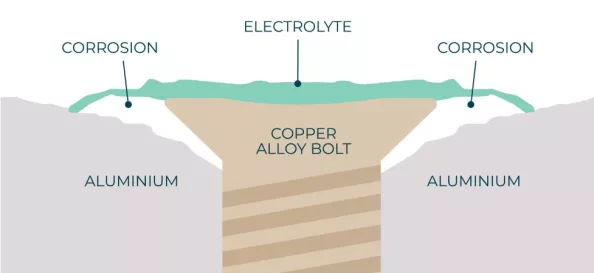
Figure showing Galvanic cell between aluminum and copper alloy bolt.

Figure showing typical galvanic series.
YouTube video explaining the Galvanic series and Galvanic cell.
https://www.youtube.com/watch?v=V3TeYfUNbGY&pp=ygUXZ2x2YW5pYyBzZXJpZXMgYW5kIGNlbGw%3D
All the Types of Metal in Engineering
Ferrous Metals: Iron and Steel Variants
1. Iron: From Pig Iron to Wrought Iron
Pig iron is the crude raw material taken directly from the blast furnace. While on the other hand wrought iron is the refined form of iron. Wrought iron typically has less carbon content of 0.08%.
| Metal Variant | Composition | Key Properties | Main Applications |
| Pig Iron |
|
|
Raw material for steel production |
| Wrought Iron |
|
|
|
Table showing ferrous metal iron.
2. Steel: Carbon Steel, Alloy Steel, and Stainless Steel
| Metal Variant | Composition | Key Properties | Main Applications |
| Carbon Steel | Iron and carbon (carbon content varies) |
|
|
| Alloy Steel | Iron alloyed with different additional elements | Tailored properties |
|
| Stainless Steel | Iron, chromium (usually at least 10-11%), nickel |
|
|
Non-Ferrous Metals: Copper, Aluminum, and Beyond
1. Copper: Properties and Copper Alloys
The precious metal copper has the chemical symbol Cu and has an atomic number of 29. Chalcopyrite (CuFeS2) and malachite (Cu2CO3(OH)2) are the raw materials for copper metal fabrication. Copper is a reddish-orange color with a high melting point, ductility, and malleability.
| Property | Value |
| Composition | Cu (atomic number 29) |
| Electrical Conductivity | Approximately 58.0 x 106 S/m |
| Thermal Conductivity | Approximately 401 W/m·K at 20°C |
| Density | Approximately 8.96 g/cm^3 |
| Melting Point | High melting point of 1,085°C (1,984°F) |
| Corrosion Resistance | Excellent |
| Malleability | Highly malleable |
| Ductility | Highly ductile |
| Antimicrobial Properties | Natural antimicrobial properties |
Table showing basic properties of copper.
Some of the copper alloys are given below:
- Bronze
- Brass
- Copper-Nickel Alloys
- Beryllium Copper
- Copper-Nickel-Silicon Alloys
2. Aluminum: Lightweight and Corrosion-Resistant
Lightweight:
Aluminum is one of the lightest engineering materials. Due to low density of 2.7 g/cm3 of aluminum it is widely used in aerospace applications. Aluminum shows a high strength to weight ratio. Its density is one-third of steel.
Corrosion Resistance:
Aluminum, when exposed to oxygen form an aluminum oxide layer on its surface. This oxide layer, also known as passivation layer act as barrier between environment and internal aluminum hence prevent it from corrosion. Aluminum is used widely in environment containing moist because of its corrosion resistance ability.
Figure showing Aluminum grade aerospace component.
3. Bronze and Brass: Copper Alloys and Their Uses
| Alloy | Composition | Common Uses | Properties |
| Bronze | Typically, 90% Cu, 10% Sn |
Marine applications Sculptures Bearings Musical instruments |
Harder More resistant to corrosion than brass Excellent for casting |
| Brass | Copper and zinc |
Plumbing fixtures Decorative items Musical instruments hardware |
More ductile and malleable than bronze Golden color |
Table showing the difference between brass and bronze.

Figure showing some non-ferrous metals.
Special Metals and Alloys
1. Titanium: Strength and Aerospace Applications
Titanium has a high strength-to-weight ratio, corrosion resistance, and high melting point making it a precious metal for aerospace use. The ultimate tensile strength of Titanium is about 434 MPa, the modulus of elasticity is almost around 105 GPa, and the yield strength of 370 MPa.
| Grade | Designation | Typical Applications |
| Grade 1 | Ti-CP1 |
Marine components Aircraft airframes Aerospace structures Aircraft engines |
| Grade 2 | Ti-CP2 |
Aircraft engines Chemical processing equipment Aircraft components Aerospace structures |
| Grade 5 | Ti-6Al-4V |
Turbine blades Aircraft components Fasteners Compressor blades Landing gear components |
| Grade 7 | Ti-0.15Pd |
Aerospace fittings Aircraft hydraulic tubing Cryogenic vessels |
| Grade 23 | Ti-6Al-4V ELI |
Aircraft structural components Dental implants Orthopedic implants Medical implants |
Table showing titanium grades that can be used in aerospace applications

Figure showing Titanium alloy 3D printed aerospace component.
2. Rare Metals and Alkaline Earth Metals
High melting points and excellent electrical properties are the major properties of Rare metals like tantalum and niobium that’s why used in different electronics and aerospace applications. Whereas alkaline earth metals are like calcium and magnesium compared to rare metals are well known for their high reactivity and are commonly used in metallurgy, pharmaceuticals, and construction applications.
| Metal | Common Elements | Main Applications |
| Rare Metals |
Niobium Zirconium Tantalum etc. |
Aerospace Electronics Nuclear reactors Superalloys |
| Alkaline Earth Metals |
Magnesium Calcium Barium Strontium |
Metallurgy Pharmaceuticals Construction Agriculture |
Table showing different elements and applications of Rare Metals and Alkaline Earth Metals.

Figure showing an image of Alkaline earth elements.
Metal Selection Criteria for Engineering Applications
Engineering materials selection can be done based on the following criteria.
- Performance Requirements.
- Reliability Requirements.
- Size, Mass, and shape Requirements.
- Cost Requirements.
- Manufacturing Requirements.
- Industry Regulations and Standards.
- Sustainability Requirements.
- Intellectual Property
Ashby’s materials selection methodology.
Engineering material selection was simplified by Ashby who has introduced engineering material selection charts which help us to assess a lot of engineering material at once comparing it with different properties.

Material selection chart comparing density and young modulus of different materials [16].
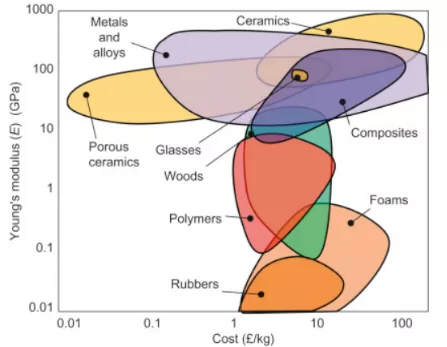
Material selection chart comparing cost and young modulus of different materials.
YouTube video on how to select materials using Ashby plots and performance indexes.
https://www.youtube.com/watch?v=9RQkvcsRzbo&pp=ygUfbWF0ZXJpYWwgc2VsZWN0aW9uIGFzYnkgYXBwcm9jaA%3D%3D
Evaluating Factors in Metal Selection
- Functionality
- Environmental Conditions.
- Engineering material properties like high strength-to-weight ratio, melting point, and durability.
- Cost.
- Testing of prototyping
- Expert Advice.
- Quality assurance
Applications of Different Metals in Engineering
1. Iron and Steel in Construction and Machinery
| Application | Iron | Steel |
| Construction |
Reinforcing bars Structural frameworks |
Structural beams, columns, roofing |
| Machinery |
Cast iron components, Machine parts |
Engine blocks Gears Tools |
Table showing applications of iron and steel in construction and machinery.
2. Copper and Its Alloys in Electrical and Thermal Applications
| Application | Copper | Copper Alloys |
| Electrical Wiring |
Electrical conductors Cables |
Brass Bronze Phosphor bronze |
| Electrical Components |
Busbars Transformers Motors |
Beryllium copper Cupronickel |
| Thermal Conductivity |
Heat sinks Heat exchangers |
Aluminum bronze Silicon bronze Nickel silver |
Table showing copper and its alloy applications
3. Stainless steel in Marine Engineering
| Application | Stainless Steel |
| Shipbuilding |
Decks Fittings Superstructures Hulls |
| Marine Equipment |
Valves Anchors Propeller shafts Pumps |
| Offshore Structures |
Platforms Subsea equipment Pipelines |
| Marine Environment |
Mooring systems Seawater desalination |
Table showing the use of stainless steel in marine components.
How metal machining services can help your project
Metal machining service helps in our project by providing precision to the fabrication of engineering materials components that can be used in different complex industrial applications. Advanced machining like CNC milling, grinding and turning to help us to fabricate metal components with great precision and accuracy. These machining services reduce production time and provide large-scale production of engineering materials components which can streamline your project .
Advanced Concepts in Metal Science
Some advanced concepts of metal science involved nanomaterials, advanced alloy development techniques, exploring metallurgy at atomic and molecular levels, novel metal fabrication methods including laser processing and additive manufacturing, and metamaterials.
Understanding Crystal Structures and Alloys
1. Body-centered cubic and Crystal Structures
In BCC atoms are present at eight corners of the cube and one atom at the center as shown in the figure below.
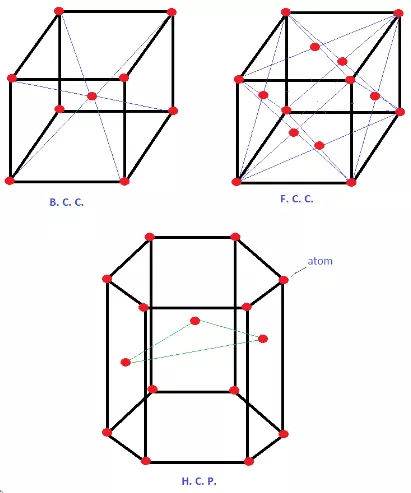
Figure illustrating the structure of BCC, FCC, and HCP crystal structures.
| Crystal Structure | Atom Arrangement | Coordination Number | Packing Efficiency | Examples | Properties |
| BCC | 8 atoms at corner and one atom at center | 8 | 68% |
Iron Chromium Tantalum |
Good ductility Moderate strength Ferromagnetic |
| FCC | Atoms positioned at the corners and 1 in the center of every face of the cube | 12 | 74% |
Aluminum Copper Gold |
High ductility High strength Good electrical Thermal conductivity |
| HCP | Hexagonal arrangement of close-packed layers of atoms each layer directly below and above | 12 | 74% |
Titanium Zinc Magnesium |
High strength-to-weight ratio Anisotropic properties High melting point |
Table showing the difference between BCC, FCC, and HCP crystal structures.
YouTube video on Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structure
https://www.youtube.com/watch?v=HCWwRh5CXYU&pp=ygUKYmNjYWNjIGhjcA%3D%3D
2. Alloy Development: From Bronze to Stainless Steel
Bronze is a historical and ancient precious metal used for corrosion resistance and aesthetic purposes but due to its easy scratch ability, softness in nature, and can’t provide corrosion resistance in a very harsh environment the new advanced alloy named stainless steel is developed which has chromium in it. Compared to bronze stainless steel provide better strength and corrosion resistance. Stainless steel provides better resistance to corrosion, scratch resistance, and strength compared to bronze.
Modern Technologies and Metal Usage
1. Electron Configuration and Magnetic Properties
Electron configuration evaluates the magnetic properties of metals. In metallurgy, we study that unpaired electron in metal dictates magnetism. Ferromagnetic types of metal like iron electron spin produce strong magnets. Paramagnetic types of metals like aluminum are weak magnets due to unpaired electrons.
2. Environmental Impact: Sustainability in Metal Production
Environmental impact concerns less engineering material usage, minimum energy consumption in metal fabrication, promoting recycling, and mitigating environmental harm in metal fabrication.
Metals in Everyday Life and Industry
Common Metals and Their Uses
| Metal | Uses |
| Aluminum | Aircraft Cans Packaging Construction |
| Copper | Electrical wiring Plumbing Electronics |
| Iron | Construction Machinery Transportation |
| Steel | Buildings Bridges Automotive Components |
| Zinc | Galvanizing Batteries Alloys |
| Lead | Batteries Radiation shielding |
| Nickel | Stainless steel metal fabrication Batteries Coins |
| Gold | Jewelry Electronics Currency |
| Silver | Jewelry Tableware Photography |
| Titanium | Aerospace components Medical implants |
| Brass | Musical instruments Plumbing fittings |
| Bronze | Sculptures Bearings Marine applications |
Table showing applications of some common metals.
Metals in Food and Health: Iron and Zinc
- Iron: is found in fortified cereals, poultry, fish, and red meat. Iron is essential for hemoglobin which helps in the transportation of oxygen in the blood and promotes cellular energy production.
- Zinc: is found in whole grains, dairy, nuts, and meat. Zinc is very important for the effective performance of immune systems, hormone regulation, and taste perception.
Industrial Manufacturing and Simple Machines
Simple machinery like levers, wheels and axles, inclined planes, pulleys, screws, and wedges plays a vital role in the manufacturing industries despite complex machinery we always need these simple machines which make our work far easier. These machineries are also the building blocks of some complex machines. The main advantage of simpler machinery is that they are easy to be used by any man don't require skilled professionals and don't require any fuel to use them compared to complex machinery.
Innovations and Future Trends in Metal Usage
1. Emerging Metal Technologies: Conductors and Corrosion Resistance
Emerging Metal Technologies for Conductors and Corrosion Resistance include superconductors, new alloys, and advanced surface treatments.
2. Metals in Renewable Energy and Automation
Metals play important roles in renewable energy technologies like electric vehicle batteries, solar panels, electric vehicle batteries, and wind turbines. Metals are added to automation systems, advanced manufacturing processes, and robotics which help in the effective fabrication of automated machinery and renewable energy systems.
Conclusion
It is concluded that the metals are essential in almost all areas of our life. From home construction to our medical health, metals play a vital role. From ancient times to modern times, the main advancement is that today the use of material is reduced, and the efficiency of material is increased. New emerging technologies like nanomaterials advanced alloy development techniques, exploring metallurgy at atomic and molecular levels, novel metal fabrication methods including laser processing and additive manufacturing, and metamaterials have changed the order concepts of using metals.
FAQ
When was metal discovered?
8000 BCF metal working was developed where copper smelting was used in ancient civilizations like Mesopotamians and Egyptians.
Most widely used metal
Iron is the most widely used metal in multiple industries like transportation, construction, and transportation.
How many types of metal are there?
90 different types of metals are present in the periodic table. Different alloys are fabricated by using these 90 metals.
In general, do metals form anions or cations?
Metals are cationic they lose electrons to stabilize themselves and form metallic bonding.
 Tel/WeChat:
Tel/WeChat:  Email:
Email: 
 Home
Home
 What is Stainless Steel? Properties, Types, and Applications
What is Stainless Steel? Properties, Types, and Applications