What is the Density of Copper? Calculation and Uses
 Oct 15,2024
Oct 15,2024

Copper is one of the most valuable materials in many industries such as construction, electrical, and electronics components. Its most fundamental feature is its mass density. Understanding the metal’s behavior under different conditions and its uses for different applications is essential. The bulk density can be used to evaluate many features in metal applications. This article will explore the density of copper and its importance in different aspects of applications.
Who Discovered Copper
Sumerians and the Chaldeans were the first ones who believed in using copper for the first time in ancient Mesopotamia. They introduced the crafting knowledge of copper to ancient Egyptians. Copper is a conductive material and therefore used in majority of the electrical applications.

Copper Synonym
Copper is originated from Latin word “Cuprum” for Cyprus which was the name of Island in Rome where it was originally found.
What is Density and Why Does it Matter in Metals?
Density defines as the mass occupies by a substance per unit volume (g/cm3). Bulk density is essential because it indicates the number of atoms and arrangement and movements of electrons. If electrons are loosely bound, density would be lower. Tightly bound electrons increase the bulk density of metal.
What is the Actual Density of Copper?
Copper has an actual density of 8.960g/cm3 at room temperature. Actual density depends on the temperature and purity of a substance.
What is the current density of pure copper?
Copper has a current density of 2.9×108A/m2.
What is the density of copper in kg/m3
The specific gravity of copper is 8,960kg/m3
What is the density of copper per mL?
Copper has a density of 8.960g/mL
What is bulk density of copper wire
The bulk density of copper wire is 8,960kg/m3
How to Calculate the Theoretical density of copper?
Cooper has a face centered cubic crystal structure. The theoretical density of copper can be found out by the density formula:
![]()
Copper Alloy Density
When copper is alloyed with other elements, its density changes. The specific gravity of copper depends on the amount of alloying element added in the Copper.
Copper 110 density
It is 99.99% pure copper and has a density of 8.960g/cm3 at room temperature
Copper c10100
Copper c10100 has a density of 8.89-8.94g/cm3
Copper c10200
Copper c10200 has a density of 8.90g/cm3
Copper c12200
Copper c12200 has a density of 8.94g/cm3
Copper c14500
Copper c14500 has a density of 8.94g/cm3
Copper c17200
It is a Beryllium Copper and has a density of 8.25g/cm3
Copper c18150
Copper c18150 has a density of 8.89g/cm3
Copper c18200
Copper c18200 has a density of 8.89g/cm3
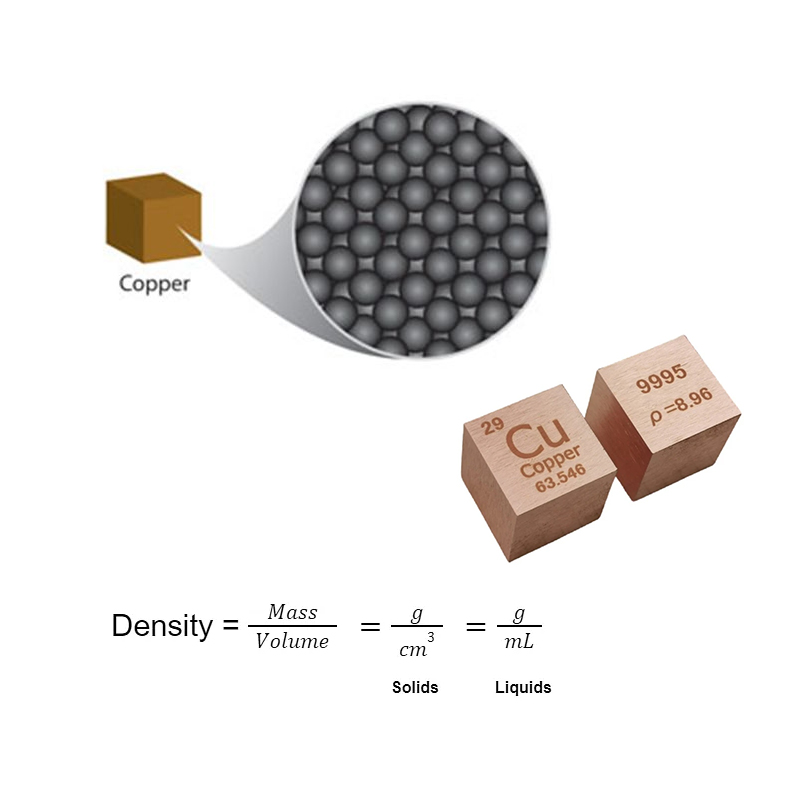
How does Copper have a High Density?
Transition metals have a high density. And copper has higher density due to two reasons. It has large atomic masses, and the arrangement of atoms is closely packed. Copper’s atomic structure is very massive.
Factors Influencing the Density of Copper
There are a few factors that can affect the density of copper
Alloying addition
Normally, when an element in copper to improve its properties, its density changes. For example, addition of zinc to make brass lowers the density of pure copper. The change in specific gravity of depends on the concentration of elements in the copper.
Processing Conditions
Manufacturing processes like drawing, casting, sheet metal fabrication changes the copper’s atomic structure and microstructure which ultimately change the density.
Temperature
Density is normally measured room temperature. If the temperature changes, it can cause thermal expansion of copper. This changes the density over contraction or expansion of copper by heat.
What is the Atomic Mass of Copper?
The atomic mass of copper is 63.54u. The electronic configuration of copper is [Ar] 4s¹3d¹⁰ and has two isotopes.
How the Density of Copper Impacts Practical Applications?
Copper has higher bulk density which is fundamental to many applications.
Electrical Applications
Copper is a conductive material, and it has most many electrical applications due to high conductivity. Its high electrical conductivity is correlated with the higher density. High density increases the atomic number and allows the electrons move freely. This ultimately makes a conductive material.
Thermal Conduction
Its high thermal conductivity also is related to the higher bulk density. Copper’s atomic structure is dense and allow high heat transfer. This increases its demand in heat exchangers and radiators along with other electrical applications.
Structural Applications
Copper’s higher bulk density is also suitable in construction and engineering. This creates a durable structure with high strength.
Copper Density in Relation to Its Thermal and Electrical Conductivity
Copper’s atomic structure is denser than aluminum. Therefore, the atomic number is greater which facilitates the electronic motion freely. This results in highly conductive material. Copper is one of the widely used materials in electrical applications. Pure copper 99.99% is the most conductive material. Heat transfer is also high in copper due to high density. Therefore, it is also used in heat exchangers and radiators.
Density variation of different copper forms
Normally, density does not change with the shape of an object. Density depends on the mass and volume of an object. It does not matter what the form of copper is, spherical, round, bar or wire, the copper’s atomic structure and density remains the same because mass and volume are constant.
Comparison of Copper Density with Other Metals
Density of Copper vs Steel
Density of cooper is slightly higher than steel. steel has 7.75 and 8.05 g/cm³ bulk density while copper has 8.96 g/cm³
Density of Copper vs Titanium
Titanium is lighter than copper with a density of 4.54 g/cm3 and copper is 8.96 g/cm³dense.
Density of Brass vs Copper
Brass is lighter than pure copper. The specific gravity of copper is 8.96 g/cm³ while brass has 8.4 g/cm³.
Density of Bronze vs Copper
Bronze has a density of 7.4-8.9 g/cm³.
Density of Copper vs Aluminum
Aluminum is 30% lighter than copper. It has 2.7 g/cm³ density
FAQ
Is Copper a Mixture?
Copper is an element. It consists of one type of atom. Therefore, it does not have any other impurity or substance and is a pure metal.
How is Copper Formed?
Copper metal is deposited from hot sulfur solutions, created in volcanic regions. The hot solutions concentrated the copper up to 1000 times more than it found in rocks. This results in enriched rocks called copper ores.
Is copper’s density suitable for heat exchangers?
Copper is a conductive material. heat transfer rate is high in copper. Heat exchangers of copper tubes have efficient energy transfer..
 Tel/WeChat:
Tel/WeChat:  Email:
Email: 
 Home
Home
 52100 Stainless Steel: A Comprehensive Guide for Engineers and Designers
52100 Stainless Steel: A Comprehensive Guide for Engineers and Designers