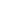Iron Carbon Phase Diagram: Definition, Phases, and Applications
 Nov 15,2024
Nov 15,2024

The Iron-Carbon Phase Diagram is super important in materials science. It shows how different temperatures & carbon amounts change the phases of iron and its alloys. When engineers get this diagram, they can make steel even better, which helps in many areas—like building stuff or flying planes.
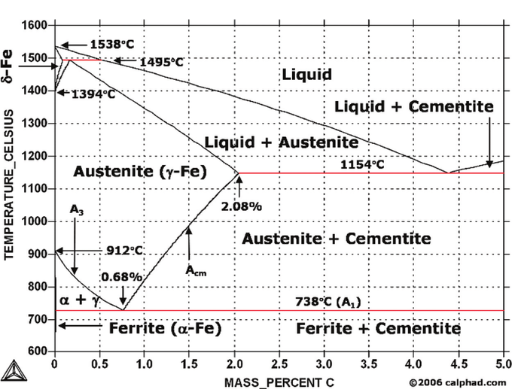
Fig 1: Fe-C phase diagram
What is the Iron-Carbon Phase Diagram?
This diagram is a chart that helps us see how temperature and carbon levels work together with iron and its alloys. It’s really helpful in metallurgy 'cause it can predict what kind of microstructure you’ll get in steel or cast iron.
What is the hardest phase in the iron-carbon diagram?
Now, martensite is the toughest phase in the Iron-Carbon Phase Diagram. This tough guy forms when austenite cools down really fast. When it cools quickly, carbon can’t escape from the iron’s structure. So, you get a solid solution that’s super hard & strong.
Why does the iron-carbon phase diagram go only to 6.7% carbon?
There’s a limit on how much carbon can be in the Iron-Carbon Phase Diagram—6.67% at most. That’s because, at that level, you make cementite (Fe₃C). If there’s more carbon than this, materials become brittle & aren't great for engineering stuff.
Phases, Phase Boundaries, Eutectic Point
Phases
Homogenous and distinct part of the system. It has different properties than other parts of the systems.
Phase Boundaries
The regions where phase transformation takes place are known as the phase boundaries. Common boundaries that we must mention are the A1 line, A3 line, and the ACM line
Eutectic Point
This point is found on the Fe-C diagram which signifies the conversion of the liquid phase into two solid phases. This transformation occurs at 1147°C with 4.3% C.
Why Phase Diagrams Are Essential in Alloy Design
These diagrams are of great importance in your everyday application and help you in determining the exact composition, properties, and microstructural changes required in your application.
Crystal Structures in Iron
Following are the prime crystal structures found in the Fe-C phase diagram:
α-Ferrite
You will find a BCC structure in alpha iron with a maximum carbon content of up to 0.02%. Moreover, if you require good magnetic properties in your application, then this phase plays a key role in that aspect.
γ-Austenite
In this phase, you will find an FCC structure with a maximum carbon content of up to 2.14%. Additionally, if your application demands any change in strength properties then this phase is important in carrying out several heat treatment processes.
δ-Ferrite
You will find this phase at elevated temperature which is around 1390°C and consists of a BCC structure. This phase will favor you in applications that require stability at high temperatures.
Cementite (Fe₃C)
This is the hardest phase in this phase diagram with maximum C content up to 6.67%. Moreover, it will benefit you in applications that require high-strength steel.
Liquid Phase (Fe-C Solution)
This phase helps the manufacturer in executing casting processes and you will find this phase at elevated temperature roughly around 1530°C.
What are the Five Solid Phases in an Iron-Carbon Diagram?
Following are the five phases you will find in this phase diagram:
- Ferrite
- Austenite
- Cementite
- Delta ferrite
- Pearlite
Characteristics of Each Phase
The below table provides you with the important characteristics of each phase:
Table 1: Phase Characteristics
|
Phase |
Crystal Structure |
Max Solubility |
Properties |
|
α-Ferrite |
BCC |
0.025% C |
Soft Ductile magnetic |
|
γ-Austenite |
FCC |
2.06% C |
Non-magnetic High toughness |
|
Cementite |
Orthorhombic |
6.67% C |
Hard Brittle |
|
δ-Ferrite |
BCC |
0.09% C |
High-temperature stability |
|
Pearlite |
Lamellar |
- |
Strength |
Temperature Ranges and Stability of Each Phase
Below are the important phases with their temperature and stability range:
α-Ferrite: This phase exists from room temperature to nearly 910°C and you will observe its stable condition below 727°C with low C.
γ-Austenite: This phase exists from 727 to nearly 1147°C and you will observe its stable condition above 727°C with high C.
Cementite: For this phase, you will find its temperature range below 1147°C and certainly stable with high C in its composition.
δ-Ferrite: You will find the formation of this phase between 1390-1538°C and favor stability at elevated temperatures.
Pearlite: You will observe the stability of this phase in eutectoid composition. Additionally, its formation occurs at 723°C.
Major Reactions in the Iron-Carbon Phase Diagram
The following reactions take place which are mentioned below:
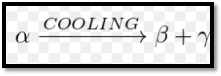
Eutectoid Reaction
Below is the eutectoid reaction which signifies you the conversion of one solid phase into a further two solid phases.
Eutectic Reaction
Below is the eutectic reaction which signifies the conversion of the liquid phase into a further two solid phases.
Peritectic Reaction
You will observe the below reaction at an elevated temperature of roughly around 1498°C which occurs by the reaction of a solid and liquid phase resulting in austenite.

Phase Boundaries and Critical Points
Below is the brief description of phase boundary and important critical points:
Understanding the Eutectic and Eutectoid Points
Eutectic point:
- Occurs at 4.3% C and a temperature of 1147°C.
- Transformation of liquid into γ and Fe₃
- Important in cast iron production.
Eutectoid Point:
- Occurs at 0.76% C and a temperature of 727°C.
- Transformation of γ into α and Fe₃
- Gives you pearlite, responsible for a blend of strength along with toughness.
Critical Temperatures in Iron-Carbon Alloys
Table 2: Critical temperatures
|
Critical temperatures |
Important transformation |
|
A1 Line (Solidus) |
γ to α and Fe₃C |
|
A3 Line |
α to Fe₃C |
|
A4 Line |
The existence of the liquid phase above this line |
|
Eutectoid Temperature |
Austenite to pearlite |
Lever Rule and Composition Analysis at Boundaries
A tool for mathematical calculation that provides you with phase proportion (mass fraction) within a specific range of temperature as well as composition.
Phase Fields and Stability Regions
The below details give you a brief analysis of phase fields and stability region:
Interpreting Stability Regions for α, γ, and δ Phases
For α-Ferrite, the phase exists from room temperature to nearly 910°C and you will observe its stable condition below 727°C with low Moreover, the γ-Austenite phase exists from 727 to nearly 1147°C and you will observe its stable condition above 727°C with high C. Finally, you will find the formation of δ phase between 1390-1538°C and favor stability at elevated temperatures.
Implications for Steel Heat Treatment
When you understand the stability region effectively, will benefit you for optimized heat treatment. Hence, crucial for phase transformation control, microstructure development along with material longevity.
Classification of Steels by Carbon Content
The major classification of steel is given below:
Hypoeutectoid and Hypereutectoid Steels
The hypoeutectoid steel gives you good ductility along with toughness in your applications and a C content of less than 0.76% is found in this steel. On the other hand, hypereutectoid steel offers you good hardness while compensating ductility and has more than 0.76% C
Role in Heat Treatment and Alloy Customization
This phase diagram offers you numerous insights, especially in heat treatment and customizing the alloy.
Predicting Mechanical Properties through Phase Analysis
Key features that help you in predicting mechanical properties through phase analysis are:
- Controlling microstructural changes
- Good understanding of critical temperatures
- Alloy properties customization
Types of Steel Applications
Table 2: Steel types & applications
|
Steel |
Applications |
|
Structural Steel |
buildings bridges |
|
Automotive Steel |
Frames engines |
|
Tool Steel |
Cutting tools Dies. |
|
Stainless Steel |
Kitchenware Medical |
|
Carbon Steel |
Pipelines Storage tanks |
|
High-Speed Steel |
Drill bits Cutting tools |
|
Mild Steel |
Beams Rods |
|
Alloy Steel |
Aerospace components Automotive |
Tuofa China Expertise in Precision Machining Steel
Tuofa Technology offers you CNC machining services that not only provide precision and accuracy but also promise excellent quality in your product. They have experience of over 16 years which further proves their expertise in the field from designing the product to final dispatching.
 Tel/WeChat:
Tel/WeChat:  Email:
Email: 
 Home
Home
 Shear Stress in Physics: Formula, Calculate, and Applications
Shear Stress in Physics: Formula, Calculate, and Applications