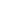What is Galvanizing: 6 Methods, Uses, Benefits
 Oct 22,2024
Oct 22,2024

Galvanizing is an ancient way of preventing corrosion, it has been widely used in the 19th century. This is the manufacturing process of applying molten zinc on the metals suspected of corrosion. If you are confused about which application or method of galvanizing is suitable. This article will provide you with enough information to choose which method is better for the subjective application.
Define Galvanizing
Galvanizing in chemistry means a simulating way to provoke an action or effort. In engineering, it means that we apply a zinc protective layer on the metals suspected of corrosion. It’s a relatively less expensive way of protection from corrosion. Prevention from corrosion is the main purpose of galvanizing. Zinc creates a barrier layer between the material’s surface and the environment to protect the material.
Different Terms of Galvanization
Since Zn is the main constituent of galvanizing, that is why it is also called zinc plating or zinc coating. Zinc plays its role as a sacrificial metal in protecting the beneath material by creating a barrier between the environmental moisture, oxygen and the metal surface.
What does Galvanization Mean?
Galvanizing in chemistry means to resist the action. In engineering, it is a metal surface treatment, and this process is used to protect metals especially steel from corrosion. It is done through six different ways which will be discussed in this article. The galvanization process is known as corrosion or rust prevention method.
Does Galvanized Steel Rust
No, galvanized steel does not rust. Zinc becomes a zinc protective layer between the environment and materials surface. This barrier layer of Zn sacrifices itself to protect the subjective material. Zn plays its role as a sacrificial anode.
The role of zinc in corrosion prevention
The role of zinc in corrosion or rust prevention method is that it sacrifices itself in preventing corrosion. In the galvanic series, a material with higher negative potential corrodes earlier. In the galvanic series, Zn has a more negative potential than steel. This process is also known as zinc treatment.

So, Zn corrodes but beneath the Zn coating material remains safe until the protective layer remains.
Can you Galvanize Over Rust?
No, a rusted surface can’t be galvanized. For galvanizing or to apply molten zinc, you need a clear surface because a rusted surface has already a layer present on it. This layer of FeO causes poor adhesion between Zn and Fe surface.
Is Galvanizing Sustainable?
Yes, you can consider galvanizing sustainability because of its long-lasting lifespan. This coating does not need any maintenance because of the self-healing effect. After completing the lifespan, both steel and zinc can be recycled and reused.
Advantages and Disadvantages of Galvanizing
The Zn protective layer is very beneficial, it provides corrosion or rust resistance. Zn is a less expensive material, which is why it is a cost-effective metal surface method to control corrosion. Galvanizing makes the material more durable.
The disadvantage of Zn coating is that the scratches on the coating make the material exposed to the environment as it can be seen in the following figure:

The other disadvantage of galvanizing is water contamination.
What is Galvanizing Used for?
Galvanizing is used for the prevention of corrosion. It increases the lifetime of the material making it more durable. Ultimately, zinc treatment increases the safety of applications. Galvanizing is used in every industry. Its common usage is protective zinc layer coated steel barriers on the sides of the road.

What is Galvanizing Steel
Galvanizing steel means protective zinc layer or coated steel to prevent corrosion. Galvanized steel is used in construction and manufacturing industries. The following figure is presenting its various applications:
When was Galvanized Steel Invented
It is an ancient way of protecting iron and steel from rust and corrosion. Galvanizing was invented in the 19th century.
What is the Cost of Galvanized Steel?
There are six zinc coating techniques which are used to apply the molten zinc on the steel. Every process has its parameters. So, the cost of galvanized steel depends on the method of galvanizing. Secondly, the thickness of the coating decides the cost.
Galvanized Steel Finish
The galvanized steel comes with a smooth, shiny and protective layer of Zn. The common appearance after galvanizing steel is a Spangled finish.

Spangles are a visible pattern of crystallites on the surface of galvanized steel that appear as snowflakes or six-pointed stars. They form when molten zinc is cooled below its melting point, causing the zinc atoms to arrange themselves in an ordered pattern.
How Galvanizing Works
In the galvanizing process, the Zn reacts with Oxygen and forms a ZnO protective layer on the material. This is a sacrificial layer which protects the base metal from corrosion. When the ZnO layer further reacts with CO2 in the moisture, ZnCO3 adheres tightly to the base metal and enhances the resistance.
Zinc as a protective layer
The zinc layer works as a sacrificial anode because it has more negative potential than iron or steel. This protective zinc layer forms a shield against moisture and oxygen.
Sacrificial anode effect
Galvanizing is a sacrificial anode effect. In electrochemical corrosion, anode and cathode are required. The anode releases the electrons and corrodes but the cathode remains uncorroded. The same is the case in galvanizing, Zn becomes an anode and steel becomes a cathode.
Longevity and durability of galvanized surfaces
Galvanized surfaces are very durable and have a longer life than uncoated surfaces. The reason is that the Zn layer can heal itself when it scratches. It reacts with O2 and forms a ZnO protective layer again.
What is the Process of Galvanizing Metal?
The basic concept of galvanizing metals (Metal Surface Treatment) is to apply a Zn coating on the base metals. Zn is applied in the form of liquid, vapors etc. depending on the method. The process starts with a metal’s surface preparation and ends with applying Zn to the metal.
6 Methods of Galvanization
In this article, 6 zinc coating techniques are discussed. The common method of zinc treatment is hot-dip galvanizing, used almost in every industry. The six methods include hot-dip galvanizing, electro-galvanizing, strip galvanizing, Sherardizing, galvannealing and mechanical plating. All these methods are suitable for different applications, let us study these methods:
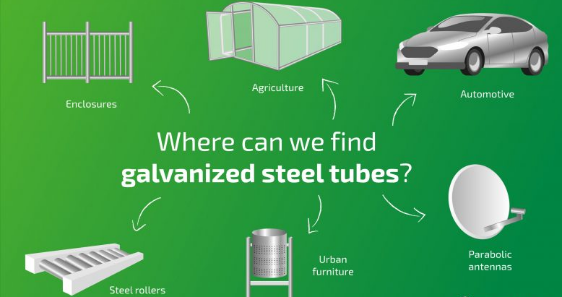
1. Hot-Dip Galvanizing
In this method, fabricated steel or any base metal is immersed into molten zinc i.e. 830ᵒF bath. This process involves three primary steps: Surface Preparation, Galvanizing and Inspection as the following figure illustrates:
2. Electro-Galvanizing
This method involves immersing a base metal into an electrically charged solution of zinc and saline. This method provides a thinner and more aesthetic protective layer of ZnO. The adhesion of the Zn layer is greater due to electric charge.
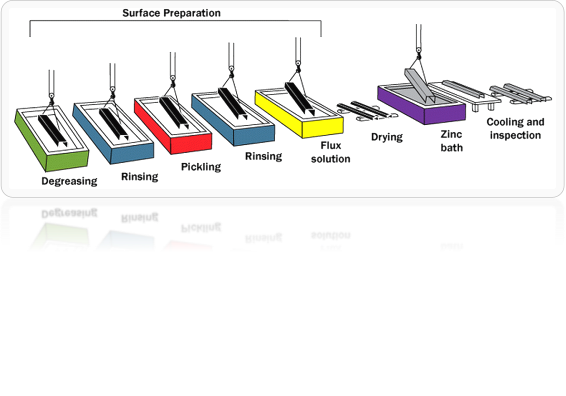
3. Strip Galvanizing
Zinc is applied to both sides of steel or iron coils when these coils pass through molten zinc. It is a continuous process. The strip-galvanized steel is further processed by forming, cutting and punching. This method provides a 7-42 microns thick layer of Zn.
4. Sherardizing
It is a diffusion coating process. Base metal is heated at 500ᵒC in a rotating drum with Zn dust or sand. The Zn evaporates at that temperature and diffuses into the surface of the base metal. It is an ideal method for small parts or parts which require internal coating. Part size depends on the size of the drum.

5. Galvannealing
It is a two-step method. In the first step, hot-dip galvanising is done while in the second step annealing is done. HDG is done at 450ᵒC while the temperature for annealing is ~556ᵒC. The final coating is harder and more brittle than galvanised steel and the colour is matt grey.

6. Mechanical Plating
Mechanical Plating is also known as peen plating or mechanical deposition. In this process of galvanizing, Zn balls are coated on the surface of the base metal through mechanical energy. It’s like cold welding at room temperature. The base metal and the coating material are placed into a fast-rotating drum. The rotating speed causes coating material to be deposited on the base metal. This method is suitable for small parts like nuts, bolts and screws etc.
Zinc Plated vs Galvanize
Both processes have the same purpose i.e. corrosion resistance. Zinc plated means zinc is applied on the base metal by an electroplating process in which both the base metal and zinc are connected to positive and negative terminals of the battery respectively. The colour of the coating is bright silver. Thickness comes to 5-25 microns.
While Galvanization has 6 methods in general to coat zinc on the base metals hot-dip galvanising is very common. In this method, the base is immersed in a molten zinc bath i.e. 450ᵒC. color of the coating is matt gray and thickness is 35 to 125 microns.
Zinc vs galvanized bolts
Zinc is just a metal like Fe. It has its own crystal structure and melting point i.e. 419.5ᵒC. It has more negative potential than any metal except Mg, which is why it corrodes very quickly. It is used as a sacrificial anode in many applications.
Galvanized bolts are zinc-plated bolts. Zinc is plated on these bolts to prevent corrosion during the application. Galvanized bolts are used in home construction projects and ornamental fencing.
Galvanized steel vs stainless steel
Galvanized steel is zinc-plated through any method of galvanizing for protection against corrosion. This zinc layer is artificial and manually applied. The thickness of the ZnO layer can vary according to the application.
Stainless steel is a steel which is covered with CrO2. When in steel formation, Cr is added into its composition. Then Cr naturally reacts with O1 to form CrO2. This layer is much stronger than zinc coating. Stainless steel is used in aggressive environments like high temperatures, chloride riches etc. The thickness of the coating is very thin and can’t be controlled.
Aluminum vs galvanized steel
Aluminum and galvanized steel both are different metals in every aspect i.e. melting point, microstructure and applications. Aluminum has its natural protective layer Al2O3. It is a highly conductive material and is used in transmission cables. Aluminum has lower strength than steel.
Galvanized steel has an artificial zinc oxide layer which is manually applied. Electrical conductivity is poor but has a higher strength and corrosion resistance than Al. The most common use of galvanized steel is as a protective barrier along the sides of roads, bridges etc.
Galvanized vs hot dipped galvanized
Galvanization is a process of deposition of Zn on the base metals to protect them from corrosion. It can be done with several methods and hot dip galvanization is one of them. In this method, a base metal sheet or part is dipped into the solution of Zn at a high temperature i.e. 450ᵒC.
Carbon steel vs galvanized steel
Both are the same steels, but the main difference is the corrosion resistance between them. Carbon steel is highly suspected of corrosion, especially intergranular corrosion. Galvanized steel has a protective layer of zinc oxide which acts as a sacrificial anode and protects the steel.
Bonderized vs galvanized
Galvanization and Bonderization methods are treatments for steel but with different purposes.
In galvanization, Zn is deposited through any process i.e. electroplating or hot-dip galvanizing method etc. for corrosion protection.
But in bonderization, the galvanized part is chemically treated with phosphate usually with chromates to increase the adhesion between the paint or base metal’s surface.
Galvanized vs powder-coated
Galvanized means zinc coated. In a powder-coated process, any paint metal is applied to steel in the form of dry powder. Then this base metal is placed into an oven, and this powder melts and gets deposited on the metal’s surface. Its purpose is for decorative or highly visible applications.
What is the Best Way to Galvanize Metal?
Overall, there are 6 methods to galvanize metals, but hot-dip galvanization is considered the best way to galvanize metals. Since, in this method, the part is dipped into zinc solution, that’s why it provides uniform coating. It is a cost-effective method relative to others, and the coating is more durable than any other method. The thickness of the coating can be tailored according to need by fixing the number of cycles of immersion.
Can You Weld Galvanized Steel?
Yes, galvanized steel can be welded but it’s difficult. Apart from health issues, when you weld galvanized steel, the coating applied on the surface becomes a barrier against penetration of the filler material. Moreover, it causes inclusions and porosity into weld.
Firstly, we need a very skilled worker to weld galvanized steel parts. Secondly, we should remove the zinc coating from the area which is expected to weld.
CNC Machining Galvanized Steel
CNC machines are very versatile and yes galvanized steel parts can be machined on CNC machines. These machines offer a clean surface finish and precise and efficient cuts.

FAQ
Galvanized Iron vs Metal Which is Stronger
Galvanization to any metal just protects from corrosion. It does not induce any strength to the base metal.
ZRC Galvanizing Compound vs Cold Galvanizing Compound
ZRC (Zinc Rich Coating) galvanizing compound contains a high content of Zn i.e. 95%. It has a unique feature of weld compatibility.
In Cold galvanizing compounds, Zn content is low i.e. 60% – 65%. It can be easily deposited through a spray or brush.
How to connect non-threaded galvanized pipe to PVC
Non-threaded galvanized pipe is connected to PVC through flexible rubber coupling. Couling plays its role as a connector. From one side, put the pipe and connect the other end of the coupling with PVC. After connection, to check the durability, pass the water through these pipes. Any leakage will indicate a poor connection.
 Tel/WeChat:
Tel/WeChat:  Email:
Email: 
 Home
Home
 Does Mild Steel Rust? Causes, Methods of Rust Removal
Does Mild Steel Rust? Causes, Methods of Rust Removal