What Are the Properties of Metalloids? Properties and Uses
 Jul 04,2024
Jul 04,2024

Metalloids are a unique category of elements. They have properties intermediate between metals and non-metals. They play a crucial role in various industries. From electronics to materials science, understanding the properties and applications of metalloids is essential. This knowledge is crucial for professionals in the manufacturing industry. In this blog post, we will delve into the characteristics, physical and chemical properties, and industrial applications of metalloids. This will provide you with valuable insights and practical advice.
What Are Metalloids?
Metalloids are elements with properties intermediate between metals and nonmetals. Metalloids are elements that exhibit both metallic and non-metallic properties. This makes them versatile and valuable in numerous applications. They are typically found along the staircase line on the periodic table. This distinguishes them from pure metals and non-metals. The significance of metalloids lies in their unique properties. These properties make them indispensable in industries such as electronics, metallurgy, and chemical manufacturing. Metals, Nonmetals, and Metalloids
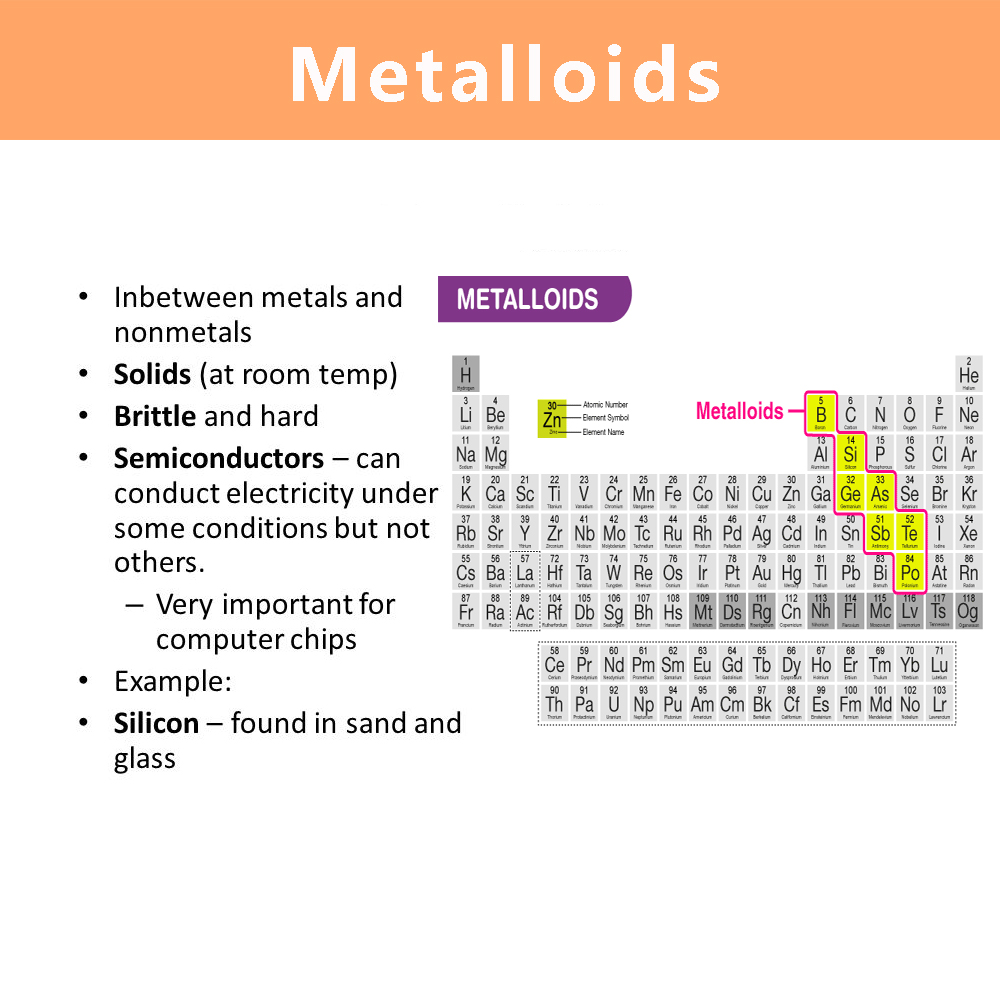
Characteristics of Metalloids
Metalloids are semiconductors, brittle, shiny or dull.
Location on the Periodic Table
Metalloids are located on the periodic table along a zig-zag line starting from boron (B) to polonium (Po). This line demarcates the boundary between metals on the left and non-metals on the right.
General Physical and Chemical Properties
Metalloids possess a mix of metallic and non-metallic properties. They are typically brittle, semi-conductive, and can form alloys with metals. Their chemical behavior varies, allowing them to act as either metals or non-metals depending on the conditions.
Common Metalloids
Common metalloids include silicon, boron, arsenic, germanium, antimony, and tellurium. They have intermediate properties, being semiconductors and useful in electronics and alloys.
|
Metalloid |
Characteristics |
|
Silicon |
Second most abundant element in the Earth's crust; excellent semi-conductive properties |
|
Boron |
Hardness, high melting point |
|
Germanium |
Critical for fiber optics and infrared optics |
|
Arsenic |
Used in pesticides and as an alloying agent |
|
Antimony |
Increases hardness and mechanical strength of alloys |
|
Tellurium |
Improves machinability of steel and copper |
|
Polonium |
Highly radioactive |
Physical Properties of Metalloids
Metalloids have a metallic luster and have intermediate electrical conductivity.
Luster and Color
Metalloids generally have a metallic luster but are not as shiny as pure metals. Their color varies, with some appearing grey or silvery.
Solid State Properties
All metalloids are solid at room temperature. They tend to be brittle, meaning they can break or shatter easily, unlike metals which are typically malleable and ductile.
Density and Hardness
Metalloids have densities and hardness values that lie between those of metals and non-metals. For instance, silicon is less dense than metals like iron. However, it is denser than non-metals such as sulfur. The densities of metalloids vary widely.For example, silicon's density is 2.33 g/cm3, while antimony's is 6.69 g/cm3. Hardness: Metalloids have a wide range of hardnesses. For example, arsenic has a Mohs hardness of 3.5, while boron has a Mohs hardness of 9.3.
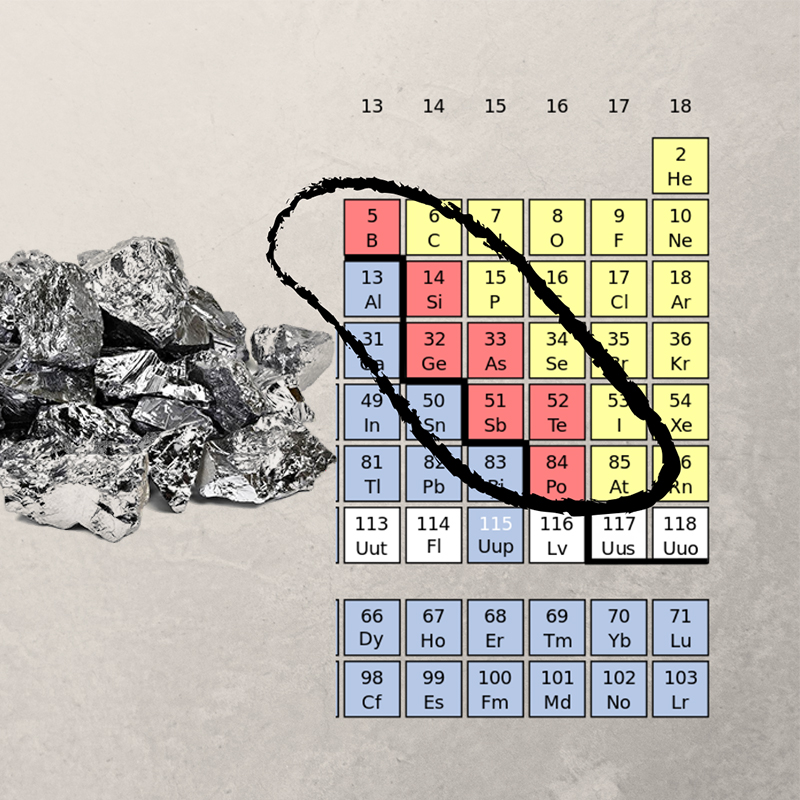
Chemical Properties of Metalloids
Metalloids have intermediate reactivity, can form amphoteric oxides, and act as semiconductors. They exhibit both acidic and basic behavior, depending on the chemical environment.
Behavior with Acids and Bases
Metalloids can react with both acids and bases, showcasing amphoteric behavior. For example, aluminum is sometimes classified as a metalloid. It reacts with both hydrochloric acid and sodium hydroxide.
Types of Bonds Formed
Metalloids typically form covalent bonds. However, they can also form ionic bonds under certain conditions. Their ability to form various types of bonds adds to their versatility in chemical reactions.
Common Compounds and Their Uses
Metalloids form numerous compounds that are essential in various industries. For example, silicon dioxide (SiO₂) is used in glassmaking and as a semiconductor material. Boron compounds are used in detergents and as insecticides.

Industrial and Technological Uses
Metalloids are used in semiconductors, solar cells, and electronics. They are crucial for making transistors, diodes, and photovoltaic cells, enhancing technological advancements.
Electronics and Semiconductors
Metalloids like silicon and germanium are crucial in the electronics industry. Silicon, in particular, is used to manufacture semiconductors. These are the building blocks of modern electronic devices.
Metallurgical Applications
Metalloids improve the properties of metals when used as alloying agents. For instance, the addition of antimony to lead enhances its hardness and mechanical strength. This makes it useful in batteries and bearings.
Chemical Industry
Metalloids play a significant role in the production of chemicals and materials. Boron, for example, is used to produce borosilicate glass, which is resistant to thermal shock.
Other Applications
Metalloids find diverse applications in different fields. For instance, tellurium is used in the production of thermoelectric devices. Silicon carbide is used as an abrasive in cutting and grinding tools.
Environmental and Health Aspects
Metalloids can be toxic in high amounts, affecting health and ecosystems. They require careful handling and disposal to prevent environmental contamination and health risks.
Environmental Impact
The mining and usage of metalloids can have environmental impacts. For example, arsenic contamination from mining activities can lead to water pollution. This affects ecosystems and human health.
Health Considerations
Many metalloids are toxic and require careful handling. Arsenic and antimony can cause serious health issues if ingested or inhaled. Proper safety measures are essential when working with these elements. Using protective equipment and following regulatory guidelines are crucial.
Summary
Metalloids have unique properties that bridge the gap between metals and non-metals. They are indispensable in various industries. Metalloids play a critical role in electronics and metallurgy. They also have applications in the chemical industry. Understanding the properties and uses of metalloids is crucial. This knowledge is essential for professionals in the manufacturing sector. Tuofa CNC machining services in China recognize this importance. As research and technology advance, the significance of metalloids is likely to grow. This will open new avenues for innovation and application.
 Tel/WeChat:
Tel/WeChat:  Email:
Email: 
 Home
Home
 Does Stainless Steel Rust? Understanding Corrosion Resistance
Does Stainless Steel Rust? Understanding Corrosion Resistance